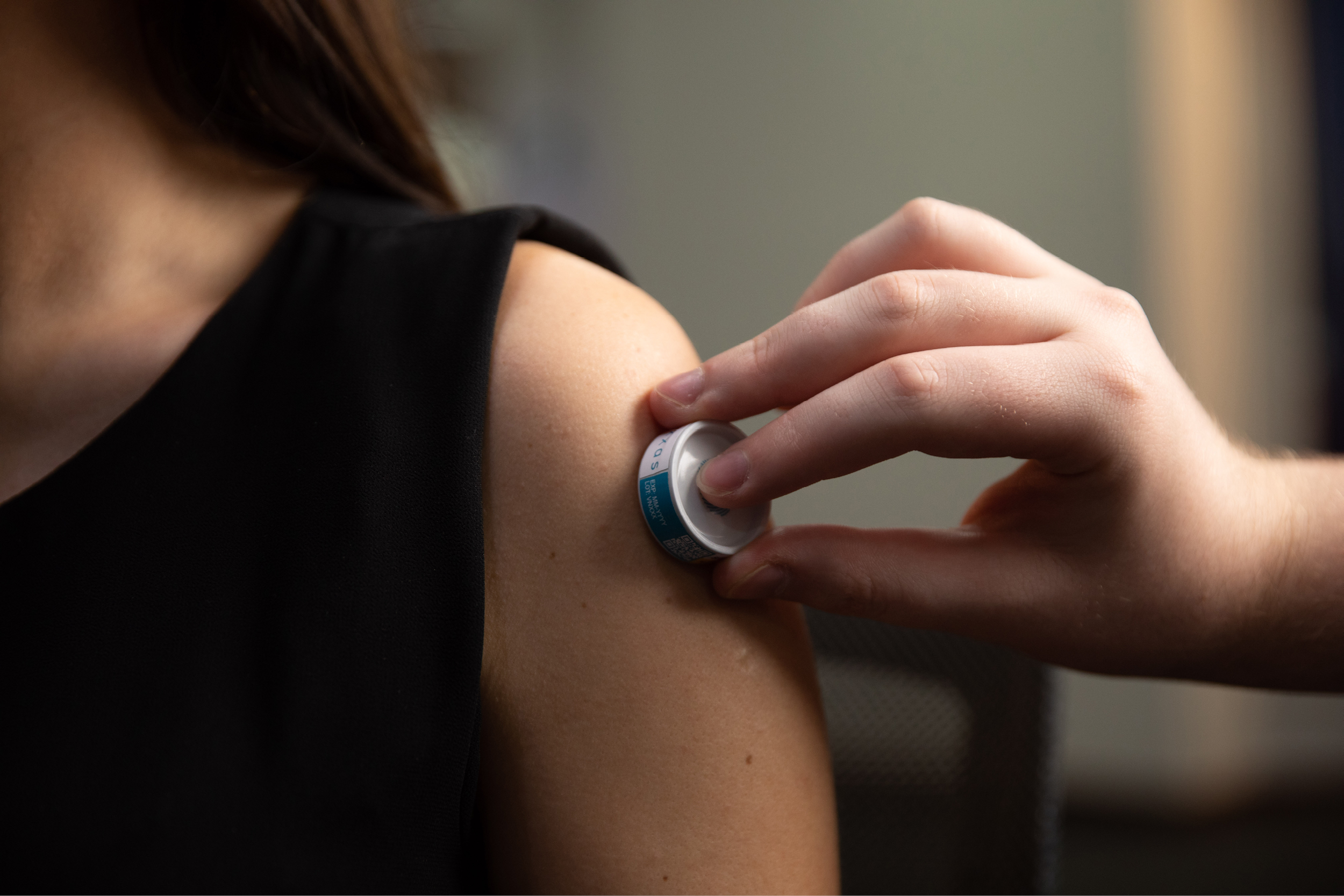
The University of the Sunshine Coast will soon begin a trial assessing Australian biotechnology company, Vaxxas’ high-density microarray patch (HD-MAP) and an investigational COVID-19 vaccine.
USC Clinical Trials has partnered with Vaxxas, developers of the high-density microarray patch (HD-MAP), after running a similar study in 2021 for measles and rubella.
Dr Angus Forster, Vaxxas Chief Technology Officer, said the study would investigate the safety and tolerability of a potential COVID-19 vaccine delivered using the company’s HD-MAP technology.
“We are pleased to be partnering again with the team at USC Clinical Trials to undertake a Phase One study of a COVID-19 vaccine using our HD-MAP technology,” Dr Forster said.
“We hope to demonstrate that delivering a COVID-19 vaccine using our HD-MAP technology can potentially be just as effective as the traditional intramuscular vaccine needle injection.”
The trial will be led by Dr Stephanie Wallace at the USC Clinical Trials clinic at Sippy Downs on the Sunshine Coast.
“It’s great to have an opportunity to work with vaccination technology that has been designed and researched here in Australia,” Dr Wallace said.
“The potential of this device to change the way vaccines are delivered around the world is very exciting.”
“This could mean that vaccines could be delivered to remote locations without the need for refrigerated transport.”
“Our community on the Sunshine Coast and at our Moreton Bay clinic have previously shown great interest in this research and have volunteered for the HD-MAP trial in its earlier phase. We would encourage them to participate again in this trial with an investigational COVID-19 vaccine,” she said.
The study requires healthy volunteers aged 18-50 years old who are in good general health and have a body mass index within the range of 18-32.
Participants will be required to visit the USC Clinical Trials clinic at Sippy Downs on the Sunshine Coast approximately seven times over a two-and-a-half-month period.
Those interested in participating can find more information at www.usc.edu.au/trials
Media enquiries: Please contact the Media Team media@usc.edu.au